CPNP Registration Services
EU Cosmetic Notification Portal
Navigate the EU cosmetic product notification portal with confidence. Expert assistance for full compliance with EU cosmetic regulations.
What Is CPNP Registration and the Cosmetic Product Notification Portal?
The CPNP (Cosmetic Product Notification Portal) is the official online system established by the European Commission for the mandatory notification of all cosmetic products before they are placed on the EU market. This centralised notification system serves as the cornerstone of cosmetic product regulation within the European Union, ensuring that every cosmetic product sold across the 27 EU member states meets stringent safety and compliance standards.
Under EU Regulation (EC) No 1223/2009, specifically Article 13, the notification portal requirement became mandatory for all cosmetic products entering the European market. This regulation fundamentally transformed how cosmetic brands, manufacturers, and importers approach EU market access, establishing a unified framework for regulatory compliance across all member states.
Key Fact: The CPNP portal is managed directly by the European Commission and serves as the single point of notification for cosmetic products sold anywhere within the European Union's internal market.
The Purpose of the Cosmetic Product Notification Portal
The cosmetic product notification portal serves multiple critical functions within the EU regulatory framework. First and foremost, it enables poison centers across EU member states to access essential product information rapidly in case of medical emergencies. When consumers experience adverse reactions or accidental ingestion of cosmetic products, medical professionals can immediately retrieve detailed formula information through the CPNP system to provide appropriate treatment.
Beyond emergency response, the notification portal facilitates market surveillance by competent authorities throughout the European Union. These authorities use the CPNP portal to verify that cosmetic products comply with EU cosmetic regulations, monitor the market for non-compliant products, and coordinate enforcement actions across borders. The system creates transparency and accountability within the cosmetics industry while protecting consumer safety.
2026 Regulatory Updates: Are You Ready?
The EU cosmetic regulatory landscape is shifting. Major updates taking full effect by 2026 include:
- Microplastics Ban: Strict restrictions on synthetic polymer microparticles in leave-on products.
- Fragrance Allergens: Expansion of the declarable allergen list from 26 to 80+ substances.
- Green Claims Directive: New substantiation requirements for "eco-friendly" and "natural" marketing claims.
- Digital Product Passport: Upcoming pilot for digital traceability of cosmetic ingredients.
Who Needs to Register on the CPNP Portal?
EU Manufacturers
If you manufacture cosmetic products within the EU, you are typically the Responsible Person and must submit the CPNP notification before placing products on the market.
Non-EU Brands (Importers)
Brands from the UK, USA, or Asia exporting to the EU must designate an EU-based Responsible Person to handle the CPNP notification on their behalf.
Distributors
Distributors may need to notify if they translate the product name/label or modify the product in a way that affects compliance (e.g., repackaging).
Responsible Person vs. Importer: Who Does What?
| Responsibility | EU Responsible Person (RP) | Importer / Distributor |
|---|---|---|
| CPNP Notification | ✓ Mandatory | No |
| PIF Maintenance | ✓ Mandatory | No |
| Safety Assessment (CPSR) | ✓ Ensures Compliance | Checks Existence |
| Label Compliance | ✓ Approves Artwork | Checks Language |
| Post-Market Surveillance | ✓ Primary Contact | Reports to RP |
Watch: Detailed breakdown of the Product Information File (PIF) requirements.
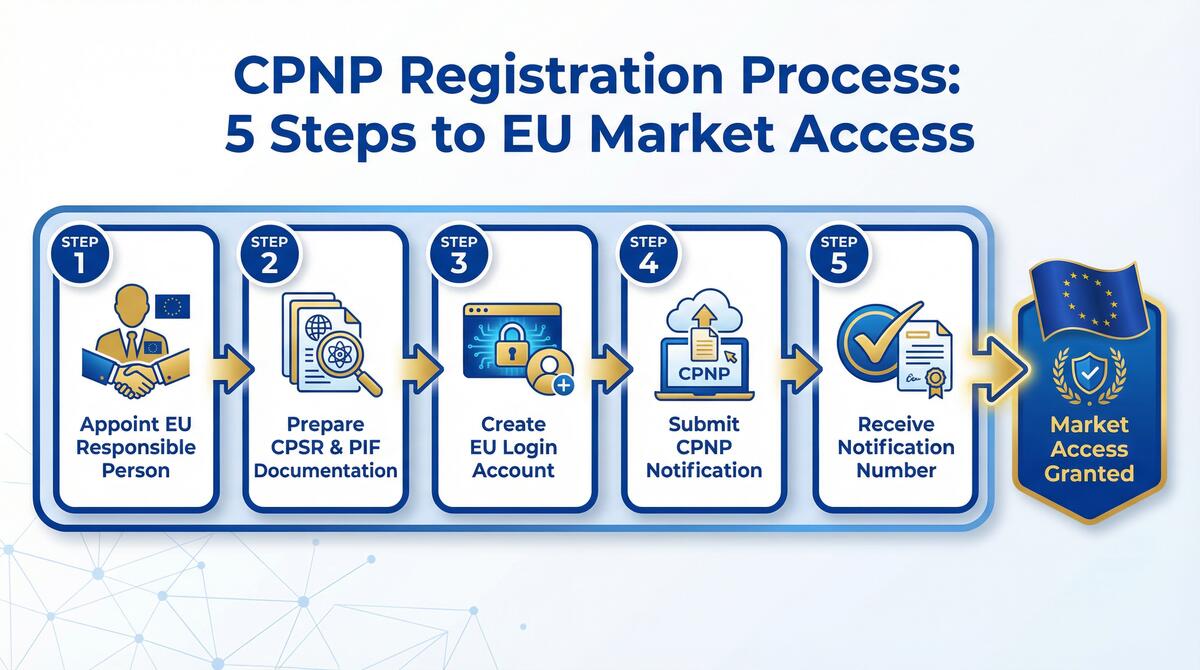
Our 5-Step CPNP Notification Process
Formula Review
We analyze your cosmetic formula (INCI list) to ensure all ingredients are permitted in the EU and within regulatory limits.
- Ingredient Compliance Check
- Restricted Substances Verification
- Part A: Safety Information
- Part B: Safety Assessment
CPSR Creation
Our qualified safety assessors prepare the Cosmetic Product Safety Report (CPSR), the mandatory safety document for every product.
PIF Compilation
We compile the full Product Information File (PIF), including GMP statements, animal testing declarations, and label artwork.
- Label Review & Translation
- Claims Substantiation
- Portal Submission
- Notification Number Generation
CPNP Notification
We submit the official notification to the CPNP portal on your behalf, generating the unique CPNP reference number for each product.
Market Access
With the notification complete and PIF on file, your products are legally compliant and ready to be sold across all 27 EU member states.
- Ongoing Compliance Monitoring
- Updates for Regulatory Changes
Typical Registration Timeline
Data Collection & Formula Review
Gathering raw material specs, MSDS, and conducting initial formula compliance checks.
Safety Assessment (CPSR)
Toxicologist reviews data and writes the Cosmetic Product Safety Report (Part A & B).
PIF Compilation & Label Review
Finalizing the Product Information File and approving label artwork for print.
CPNP Notification Submission
Official submission to the portal. Product can be marketed immediately after notification.
Estimated Turnaround Times:
- 🚀 Best Case: 10 Days (All data ready)
- 📅 Typical Case: 3 Weeks (Standard process)
- ⚠️ Worst Case: 3-6 Months (Testing needed)
Pro Tip: If stability testing is required (for new formulas), add 3 months to your timeline. Start early!
Transparent Pricing Packages
Detailed Component Pricing
Transparent breakdown of individual service costs
Core Services
- EU Responsible Person €500 - €1,200 / year
- CPNP Registration Support €50 - €150 / product
- CPSR (Safety Report) €180 - €450 / product
- PIF Compilation €100 - €300
Additional Testing (If Needed)
- Stability Testing €200 - €500
- Challenge Testing (PET) €150 - €400
- Microbiological Testing €100 - €300
- Label Translation €50 - €150 / language
Starter
Ideal for 1-5 Products
- EU Responsible Person (1yr)
- CPSR Preparation (Part A & B)
- CPNP Notification Submission
- PIF Setup & Maintenance
- Basic Label Review
Growth
Ideal for 6-15 Products
- All Starter Services
- Volume CPSR Discount
- Priority Support (24h)
- Comprehensive Label Review
- Claims Substantiation Check
Enterprise
16+ Products & Complex Needs
- Full Compliance Package
- Dedicated Account Manager
- EU + UK Dual Registration
- Ongoing Regulatory Updates
- Crisis Management Support
Consequences of Non-Compliance
Failing to comply with EU cosmetic regulations and CPNP notification requirements carries serious consequences. Competent authorities across the EU actively enforce these regulations.
Financial Penalties
Fines typically range from €5,000 to €50,000+ per product depending on severity and member state.
Market Removal & Seizures
Authorities can seize non-compliant products, mandate recalls, and destroy inventory at your expense.
Marketplace Suspensions
Amazon, eBay, and other platforms suspend accounts that cannot prove CPNP compliance.
Risk Warning
In serious cases involving consumer harm or deliberate fraud, criminal prosecution is possible. Responsible persons can face personal liability for compliance failures.
Compliance failures become public through RAPEX alerts, damaging brand reputation permanently. Building a reputation for compliance is far easier than rebuilding trust after violations.
California CBD Skincare Brand Enters EU Market
From Amazon Suspension to €180k Revenue in Year 1
The Challenge
A California-based CBD skincare brand with 3 SKUs attempted to list on Amazon.de but was immediately suspended for lack of CPNP notification and Responsible Person. They risked losing their entire European expansion budget.
The Solution
- Appointed us as EU Responsible Person (Dublin office)
- Conducted full formula review for CBD compliance (0.0% THC)
- Prepared CPSR Part A & B for all 3 products
- Completed CPNP notification within 3 weeks
The Outcome
Amazon account reinstated within 48 hours of document submission. The brand generated €180,000 in EU sales in the first 12 months.
Frequently Asked Questions
Get Your Free Compliance Quote
Complete the form below to receive a personalised quote for your cosmetic product notification needs. We respond within 24 hours.